Anterior Segment Disorders and Dry Eye
The Cheng Lab
The Cheng Lab aims to understand the mechanisms for establishing and maintaining lifelong homeostasis and transparency in the eye lens. While decades of studies have identified the genes required to make a transparent lens, there remain many unanswered questions about basic lens cell biology that hinder the development of pharmaceuticals to prevent or delay age-related lens pathologies, including cataracts and presbyopia. 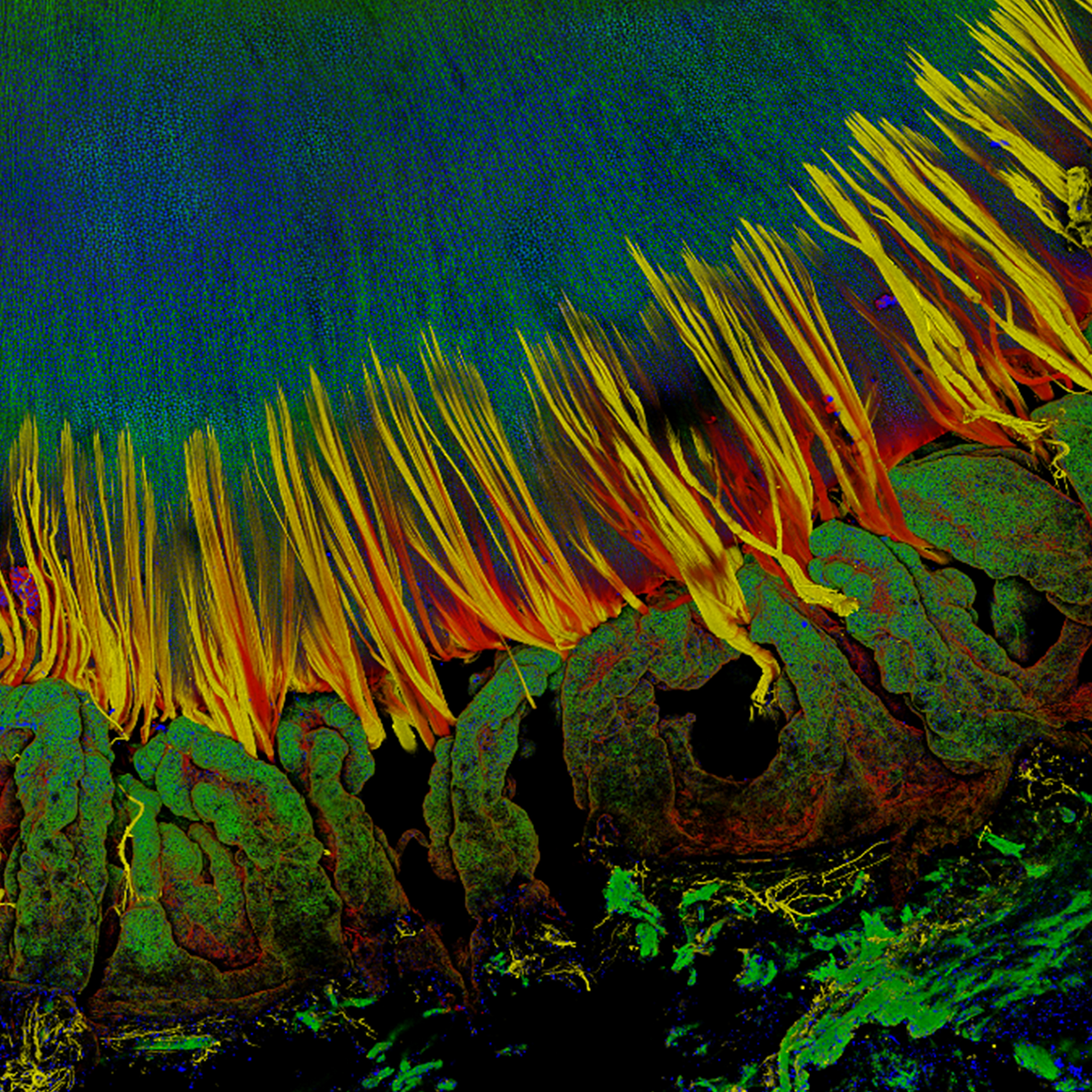
In humans, ciliary muscles contract to change the tension of zonule fibers to deform the lens, increasing the len's optical power to focus on nearby objects, a process known as accommodation. This image shows a 3D reconstruction of a confocal z-stack through a human lens with attached ciliary body and zonules. The zonules are stained with wheat germ agglutinin (cell membrane marker, red) and microfibril-associated glycoprotein MAGP-1 (zonules, yellow). The ciliary body (in the foreground of the image) and the lens (behind the zonules) are stained for filamentous actin (F-actin, phalloidin, green) and cell nuclei (DAPI, blue)
Visit the Cheng Lab Website
Frontiers of Optical Imaging and Biology Lab
Patrice Tankam, Ph.D.
Optical imaging techniques such as optical coherence tomography (OCT) and fluorescence microscopy (FM) have enabled tremendous discoveries in biological and clinical research. However, the need for enhancing technological innovations to enable a better understanding of cellular processes involved in developmental and pathological conditions is still manifest. Dr. Tankam's lab uses a translational research approach that spans from the mechanistic understanding of corneal diseases using transgenic mice to disease diagnosis and management in humans. The group focuses on developing advanced imaging systems, including an integrated cellular-resolution OCT and FM to track the dynamics of cellular processes in vivo in the same transgenic mouse over time, as well as cellular-resolution OCT systems for human corneal imaging. The group is particularly interested in understanding the structural and physiological remodeling of the cornea and the iridocorneal angle in pathological conditions such as myopia, Fuchs endothelial corneal dystrophy, and primary open angle glaucoma.
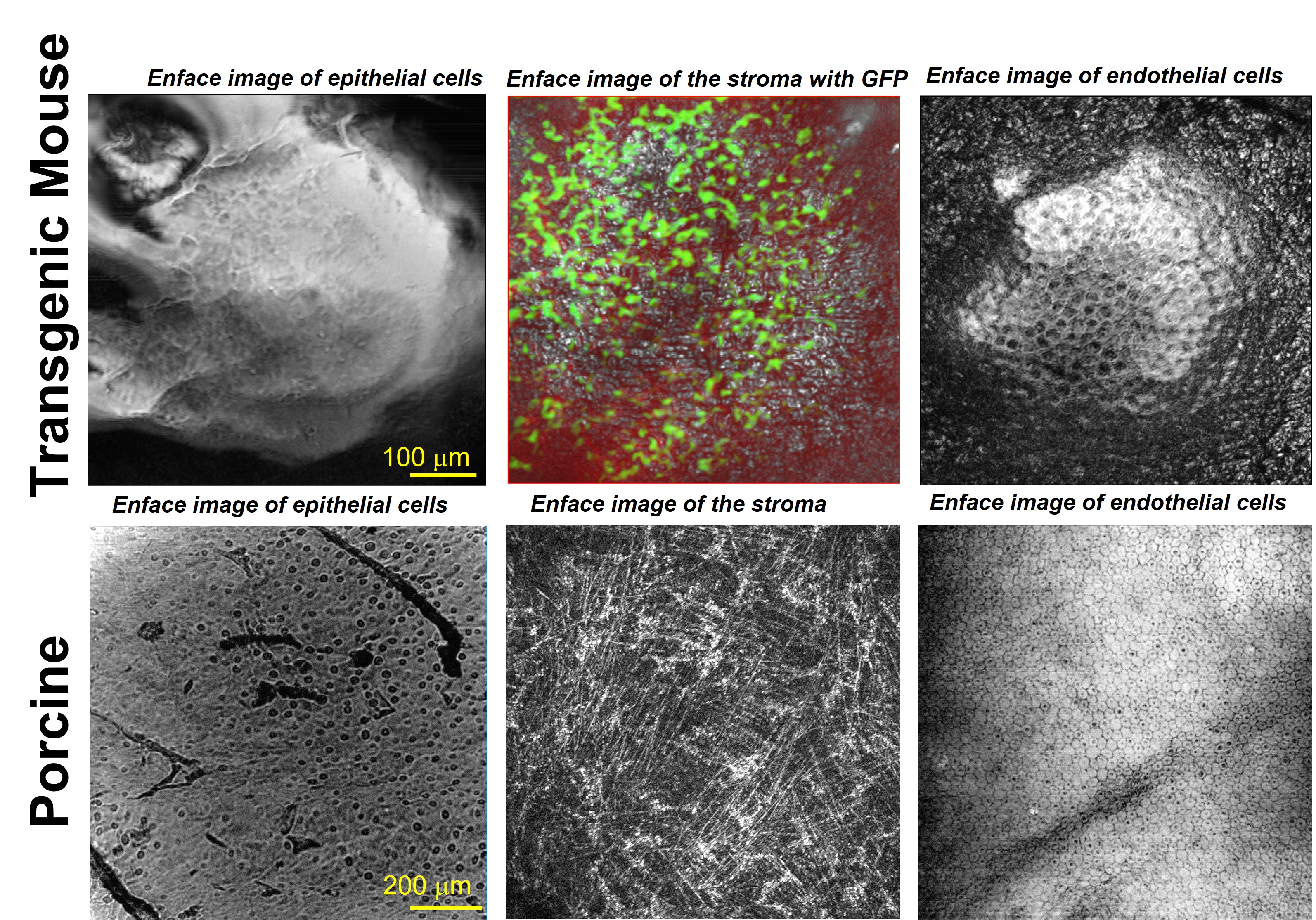
Corneal Imaging with Cellular-resolution Optical Coherence Tomography
Visit the Frontiers of Optical Imaging and Biology Lab Website
Our main goal is to advance the understanding of the neurological basis of dry eye disease using a combination of psychophysical, physiological and imaging approaches. My primary research interests include A) sensory function of the ocular surface and its role in dry eye disease and contact lens discomfort, B) psychophysical assessment of ocular surface sensation and pain, and C) the impact of tear film dynamics on ocular surface sensation.
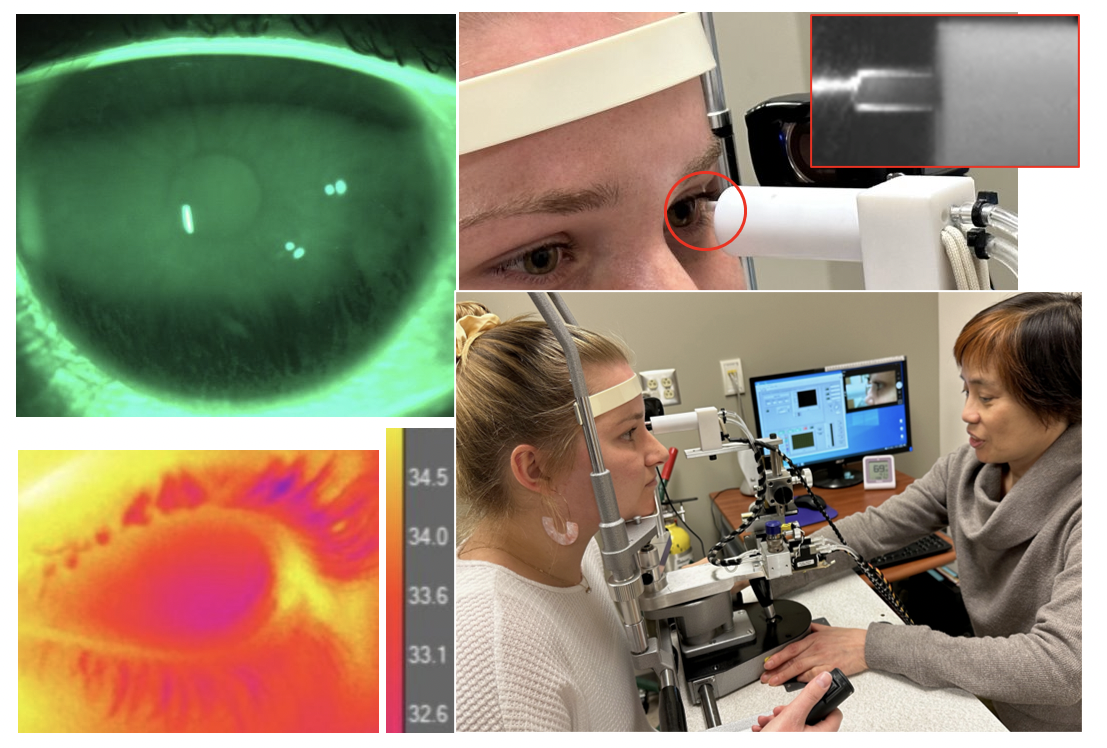
Imaging tear film dynamics.
The corneal endothelium is responsible for maintaining the hydration of the cornea. This function is essential for good vision since a defective endothelium will lead to a reduction in corneal transparency. Bonanno/Ogando lab research endeavors to uncover the transport processes underlying the “Corneal Endothelial Pump”, loss of endothelial cells & function due to aging or endothelial dystrophies, and metabolic processes for maintaining endothelial health in order that medical treatments for endothelial dysfunction can be developed.

Histological cross section of the human cornea.
Three major layers: 1) Epithelium faces the tears, 2) Stroma contains keratocytes that produce collagen and GAGS (Glycosaminoglycans), and 3) posterior surface Endothelium facing the anterior chamber aqueous humor. GAGS draw water into the stroma primarily across the endothelium. An equal amount is removed by active transport processes of the Endothelial Pump. This yields a steady-state stromal hydration, transparency, and clear vision.

